Electrosynthesis Exchange
Learning Resources
M. C. Leech, K. Lam*
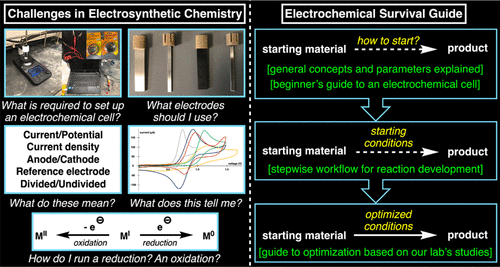
This paper discusses the role of each component of the cell (electrodes, solvent, electrolyte, etc), with cases studies demonstrating the importance that each of these can have. This is of interest when working out the practicalities of a reaction design, such as the solvent choice.
Keywords: Tutorial, Theory, Troubleshooting, Case study
M. Rafiee*, M. N. Mayer, B. T. Punchihewa, M. R. Mumau
This paper provides a discussion of the four basic types of bulk electrolysis (constant current and constant potential, each with or without reference electrode variations). Explanations of the principles of each technique as well as tips and illustrated applications. are presented. This is of interest when deciding which mode of electrolysis to use when designing an experiment.
C. Schotten, T. P. Nicholls, R. A. Bourne, N. Kapur, B. N. Nguyen, C. E. Willans*
This paper provides an overview and mathematical context for the concepts for electroroganic synthesis. Lots of practical information such as, a table of "pros and cons" of electrodes and of electrolysis modes, a chart for common redox potential of organic moeties, and potential windows of solvent/electrolyte pairs. A convenient guide that evaluates potential impacts for each optimization parameter is also provided, as well as a FAQ for troubleshooting. This paper is of interest for designing and optimizing electrochemical reactions, and also for troubleshooting issues.
G. Hilt*
This paper provide a discussion of various types of bulk electrolysis (alternating polarity, cation pool), as well as other considerations (divided vs undivided, direct vs mediated). It provides rational for the choice of each setup and examples displaying where they solved a problem. This paper is useful when designing an electrochemical experemiment.